Tiny
‘molecular motors’ shed light on how cells carry out
their functions
Interdisciplinary approach blended physics and
biology to arrive at explanation for cellular order
By MITZI BAKER
Every cell in the body has what James Spudich,
PhD, calls “a dynamic city plan” comprised of molecular
highways, construction crews, street signs, motor cars, fuel and
exhaust.
Maintenance of this highly organized structure is fundamental to
the development and function of all cells, Spudich says, and much
of it can be understood by figuring out how molecular motors do the
work to keep cells orderly.
Spudich, biochemistry professor at the School of Medicine, and
Stanford physics graduate student David M. Altman reported in the
March 5 issue of Cell how a type of molecular motor
provides the rigidity needed by the tiny sensors in the inner ear
to respond to sound. They found that this motor creates the proper
amount of tension in the sensors and anchors itself to maintain
that tension.
“Our general feeling is that tension-sensitive machines are
at the heart of the dynamic city plan,” said Spudich.
Their National Institutes of Health-funded study has implications
far beyond how an obscure molecule provides rigidity for a protein
in the inner ear. A motor able to create structural changes by
taking up slack in proteins and clamping down so that they remain
in a rigid position may help explain many intricacies of cellular
organization, such as how chromosomes line up and separate during
cell division.
“Studies like this allow you to understand enough details of
these motors to design small molecules to affect their
function,” said Spudich, who is also the Douglass M. and Nola
Leishman Professor of Cardiovascular Disease. Toward this end he
has co-founded a company, Cytokinetics, in hopes of creating drugs
that selectively target molecular motors involved in cancer and
cardiovascular disease.
For years, Spudich’s lab has studied molecular motors called
myosins, proteins that carry out cellular motion by attaching to
and “walking” along fibers of actin. The interaction of
actin and myosin is the mechanism behind cell actions such as
muscle contractions, the pinching off of two daughter cells from a
mother cell during division and the hauling of cargo molecules
around in a cell.
Of the 18 types of myosin molecules, their current findings examine
myosin VI, thought to be responsible for setting the tension for
stereocilia, actin-filled rods on the sound-sensing hair cells of
the inner ear. A defect in myosin VI results in deafness.
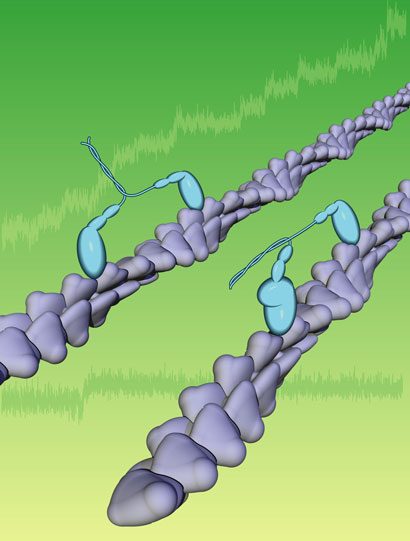
A myosin
molecular motor attaches to a portion of an actin filament and
walks down its length. If the myosin tail is carrying a load, it
stops walking and turns into a clamp when a certain level of
tension is reached. Researchers think that this clamping mechanism
can explain much of how cells maintain their internal structural
organization. Illustration:
Courtesy of Spudich Lab
Although it was known that myosin moves along actin fibers, it had
never previously been demonstrated how myosin could function as an
anchor or a clamp. To study this, Spudich and Altman needed
techniques beyond the realm of biology.
“This is a problem for physicists who think in terms of
forces and putting a load on a system,” said Spudich. Altman
specializes in optical tweezers, a focused laser that allows the
manipulation of microscopic beads, and provided the required
physics know-how by applying his expertise to studying myosin
activity precisely.
The Cell paper includes a number of complex equations
describing how the myosin VI anchor works, but the researchers have
easily simplified the concept: think of the palm of an open hand as
the hair cell and the fingers as the stereocilia. Myosin VI has two
legs as well as a tail, which can bind to other things.
The researchers think the myosin VI tail in the hair cell binds to
the webbing between the fingers – the cell membrane between
the stereocilia – and then as the legs walk across the palm
(the hair cell) it pulls the webbing between the fingers taut which
makes the stereocilia rigid.
As the motor continues walking, the taut membrane strains the motor
and distorts its shape, which turns the motor into an anchor. If
the webbing/membrane becomes slack again, the motor regains its
normal shape and begins walking again. It continues walking until
the membrane becomes taut again.
“You can imagine that if a motor like this didn’t
stall, it would end up continuing to burn energy in the cell and
would keep pulling this membrane, but it would be wasting a lot of
energy,” said Altman, who is first author of the paper.
“So this change has made it a smart and efficient
motor.”
“The sophistication of what David has been able to do here in
terms of looking at a single molecule and how it behaves is
unusual,” Spudich noted. “There are very few proteins
in biology that have been analyzed and understood down to this
level.”
Altman is now looking at defective myosin VI that causes deafness
in hopes of learning even more about the precise refinement of the
molecular motor.
Studies of molecular motors are fundamental to understanding all of
cell biology, said Spudich, and require a multi-faceted approach
combining the input of several disciplines.

|

