|
Researcher
wishes estrogen study went a year longer
By SUSAN IPAKTCHIAN
Publishing the results of a nearly seven-year Women's Health
Initiative study dealing with estrogen therapy is bittersweet for
Marcia Stefanick, PhD, professor (research) of medicine at the
Stanford Prevention Research Center and chair of the WHI national
steering committee.
While Stefanick is pleased that the results provide clear evidence
that initiating unopposed estrogen therapy for women in their 60s
and 70s is not beneficial, she's disappointed that a decision to
end the study a year early means that answers aren't as precise for
women in their 50s.
"I think it puts exactly the same questions back on the table that
were there when we started the trial for women in their 50s,"
Stefanick said. "We probably could have answered them if we had
been given the full eight years."
The estrogen therapy tria l-- involving nearly 11,000 women
nationally between the ages of 50 and 79 who had undergone a
hysterectomy -- was halted at the end of February because of
concerns that estrogen increased the risk of stroke while offering
no protection against heart disease. The initial study results are
published in today's issue of the Journal of the American
Medical Association.
Graph: Courtesy of Marcia
Stefanick
Although the primary purpose of hormone therapy is to help relieve
the effects of menopause, observational studies and other evidence
over the years have suggested that hormones might prevent heart
disease and bolster the overall health of older women. The WHI
study wanted to answer those questions definitively.
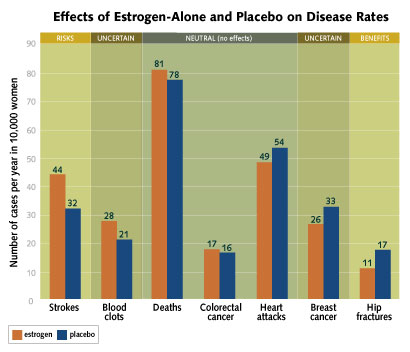
Overall, the results show that the estrogen used in the study,
known as conjugated equine estrogen, doesn't appear to have any
effect on the risk of heart attacks, colorectal cancer or deaths.
The study showed a 39 percent increase in the risk of stroke, going
from 32 strokes per 10,000 women among those taking a placebo to 44
strokes per 10,000 women among those on estrogen. The one clear
benefit of estrogen was in a decreased risk of hip fracture. Two
areas in which estrogen's effect is uncertain are breast cancer and
blood clots. Although fewer women on estrogen developed breast
cancer, researchers said the result was not statistically
significant.
But when the results are broken down by age group, Stefanick said,
the findings aren't as clear-cut for women in their 50s. For them,
estrogen appears to reduce the risk of heart disease and the
overall picture suggests benefit.
"For women in their 60s and 70s, I think the message is pretty
clear that initiating hormones is not beneficial for preventing
heart disease or for their overall health," Stefanick said. "But
for women in their 50s who have had a hysterectomy, this study does
suggest some benefit. We would have had better information if we
could have continued the trial for another year."
The decision to halt the trial was made by the National Institutes
of Health. Although none of the previously established boundaries
for stopping the study was crossed, the NIH felt that the increased
risk of stroke was not acceptable in healthy women in the absence
of any benefit to heart disease. While Stefanick acknowledged that
concern, she was unclear as to whether it outweighed the benefit of
continuing the trial for another year to give women more precise
answers about the balance of risks and benefits of estrogen.
"We're in the unfortunate position of having stopped the trial
because of the risk of stroke, which occurred primarily in women in
their 60s and 70s," she said. "The study came up short for women in
their 50s."
Stefanick said the early end of the trial also makes it difficult
to know how long women can safely continue taking estrogen. In the
findings related to heart disease, the results showed that women on
estrogen had more heart attacks in the initial years but Stefanick
said this effect diminished over time in a significant way.
"While we would encourage older women not to initiate hormone use,
there's nothing from this study to suggest that they should stop
taking it if they've already been on it for up to seven years," she
said.
For women in their 50s who are contemplating whether to initiate
estrogen, Stefanick said they should discuss their questions with
their physicians. "There's certainly good evidence that it's not
harmful for women in their 50s who have had a hysterectomy and
would only take estrogen for at least seven years."
The message for all women and their physicians is that hormone
therapy should be used for relief of hot flashes and other symptoms
related to menopause and not as a method of preventing disease,
Stefanick said. The Food and Drug Administration recommends that
women use the lowest hormone dose needed to achieve treatment goals
and limit the duration of the therapy.
Stefanick said she and the other WHI investigators will continue
analyzing the data in the coming months and will publish more
detailed results in the fall. The study also has received funding
to continue tracking clinical outcomes in the women through
2010.
WHI was launched in 1991 to examine the most common causes of
death, disability and impaired quality of life in postmenopausal
women. The 15-year, multimillion-dollar effort involves more than
161,000 women nationwide.
In addition to the study of estrogen and an earlier study of the
combination of estrogen and progestin, the study has arms examining
the role of a low-fat diet in reducing breast and colon cancer; the
role of calcium and vitamin D in fracture prevention; and an
observational study to identify disease predictors. Those arms are
continuing.
In 2002, the arm dealing with the estrogen-progestin combination
was halted because of evidence that the participants experienced a
greater risk of breast cancer, heart attack, stroke and blood
clots.

|

